Abstract
Background Bispecific CAR-T cell therapy has been proposed to mitigate some limitations of single-targeted CAR-T cells. CS1 plays a vital role in myeloma pathogenesis, and is also highly expressed on tumor cells in almost all patients (pts) with multiple myeloma (MM). Therefore, we aim to augment BCMA targeting with CS1. Bispecific CS1-BCMA CAR-T cells are effective in targeting MM cells in preclinical studies (Biomedicines 2021). Here we report the outcomes of 16 pts with refractory or relapsed (RR) MM in our phase I clinical trial (NCT04662099).
Methods CS1-BCMA bispecific CAR contains a murine anti-CS1 scFv (clone 7A8D5) and a murine anti-BCMA scFv (clone 4C8) in tandem, and a 4-1BB costimulatory domain. The enrolled pts must have received at least 2 prior lines of therapy, and previous BCMA- or CS1-targeted immunotherapies were allowed. Pts were subjected to lymphodepleting regimens with cyclophosphamide (250 mg/m2, d-5 to d-3) and fludarabine (30 mg/m2, d-5 to d-3) daily prior to the CAR-T infusion (d0). Planned dose levels were 0.75x106, 1.5x106, and 3.0x106 CAR+T cells/kg, and repeated infusions were allowed. Primary objectives were incidence of adverse events (AEs). Cytokine release syndrome (CRS) and neurotoxicity were graded using the ASTCT criteria, and other AEs using CTCAE v5.0. Secondary objectives were overall response rate (ORR), overall survival (OS), duration of response (DOR), and progress-free survival (PFS). Response was assessed per the IMWG criteria 2016. Other objectives included in vivo kinetics of CAR-T cells, changes of soluble BCMA, immune assessment of infused CAR-T cells and patients’ lymphoid and immunosuppressive phenotype before and after infusion.
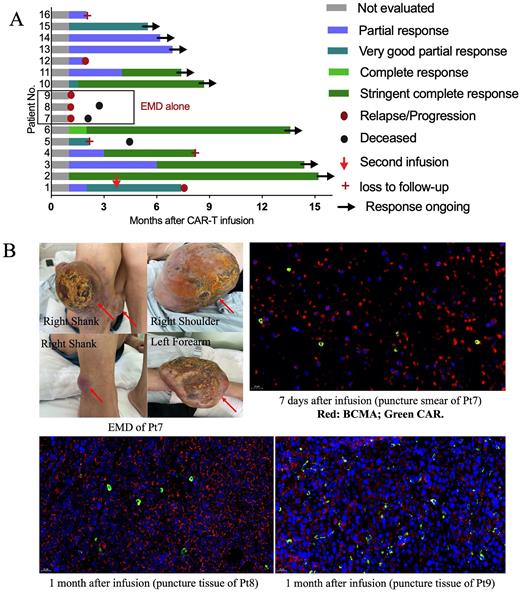
Results As of July 31, 2022, 16 patients received the infusion of CS1-BCMA CAR-T cells and were included in the final analysis. Nine pts (56%) carried cytogenetic abnormalities, and 3 pts had extramedullary diseases (EMD) alone without detectable MM cells in bone marrow (BM). BCMA and CS1 were highly expressed on MM cells in BM by flow cytometry (mean BCMA+76.7% and CS1+ 96.5%) and EMD by trichromatic immunofluorescence. Nine pts (56%) were refractory, and 7 pts (44%) were relapsed and refractory. The median prior lines of treatment were 5.4 (range 2-10). All pts (100%) were exposed to bortezomib, 6 (38%) to ixazomib and 1 (6%) to cafilzomib. 13 pts (81%) received lenalidomide, 6 (38%) received thalidomide and 5 (31%) received pomadomide. 7 pts (44%) received autologous transplantation, 6 (38%) were exposed to daratumumab, 2 (13%) received selinexor and 2 (13%) had previous BCMA-targeted CAR-T therapy.
Six pts (38%) experienced CRS, with median duration of 4 days (range 4-7). Grade 3 CRS occurred once and lasted for 5 days. Neurotoxicity was not observed. The ORR was 81% (13/16) for all patients, including 6 stringent complete, 3 very good partial, and 4 partial responses (Fig.1A). The ORR was 100% (13/13) for patients with MM cells in BM. Among them, 7 pts (54%) maintained ongoing response, 2 pts progressed after response, 1 withdraw from the study and 3 were lost due to poor compliance. Pt 16 had received prior anti-BCMA CAR-T treatment and achieved a partial response after CS1-BCMA CAR-T infusion. Although CAR-T cells infiltrated into the tumor tissue, response was not observed in the 3 pts with EMD alone (Fig.1B). Soluble BCMA decreased remarkably in peripheral blood (PB) and BM after infusion. At a median follow-up of 290 days, the median OS, PFS and DOR weren't reached, and OS, PFS and DOR at 12-month were 83.9%, 55.2% and 68.8%, respectively.
On day 14 after infusion, CAR-T cells peaked at 138245 copies/ug DNA in PB (n=16) and 86654 copies/ug DNA in BM (n=13) by droplet digital PCR; CAR-T cells peaked at 493 CAR+T cells/ul PB (n=16) and 312 CAR+T cells/ul BM (n=13) by flow cytometry. CAR was detectable in vivo at a median of 6 months. Because CS1 is expressed on T and NK cells, we systematically evaluated the immune characteristics (differentiation, Fas, LAG3, PD1, TIM3, CS1) of infused products, absolute quantification and CS1 expression on T and NK cells in PB as well as myeloid derived suppressor cells, regulatory B and T cells in BM and PB. Further data will be released in the meeting.
Conclusions Our study demonstrates bispecific CS1-BCMA CAR-T cells are clinical active with a good safety profile in heavily pretreated pts with MM, even after BCMA-targeted CAR-T cell therapy. Further studies are needed for pts with EMD alone.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal